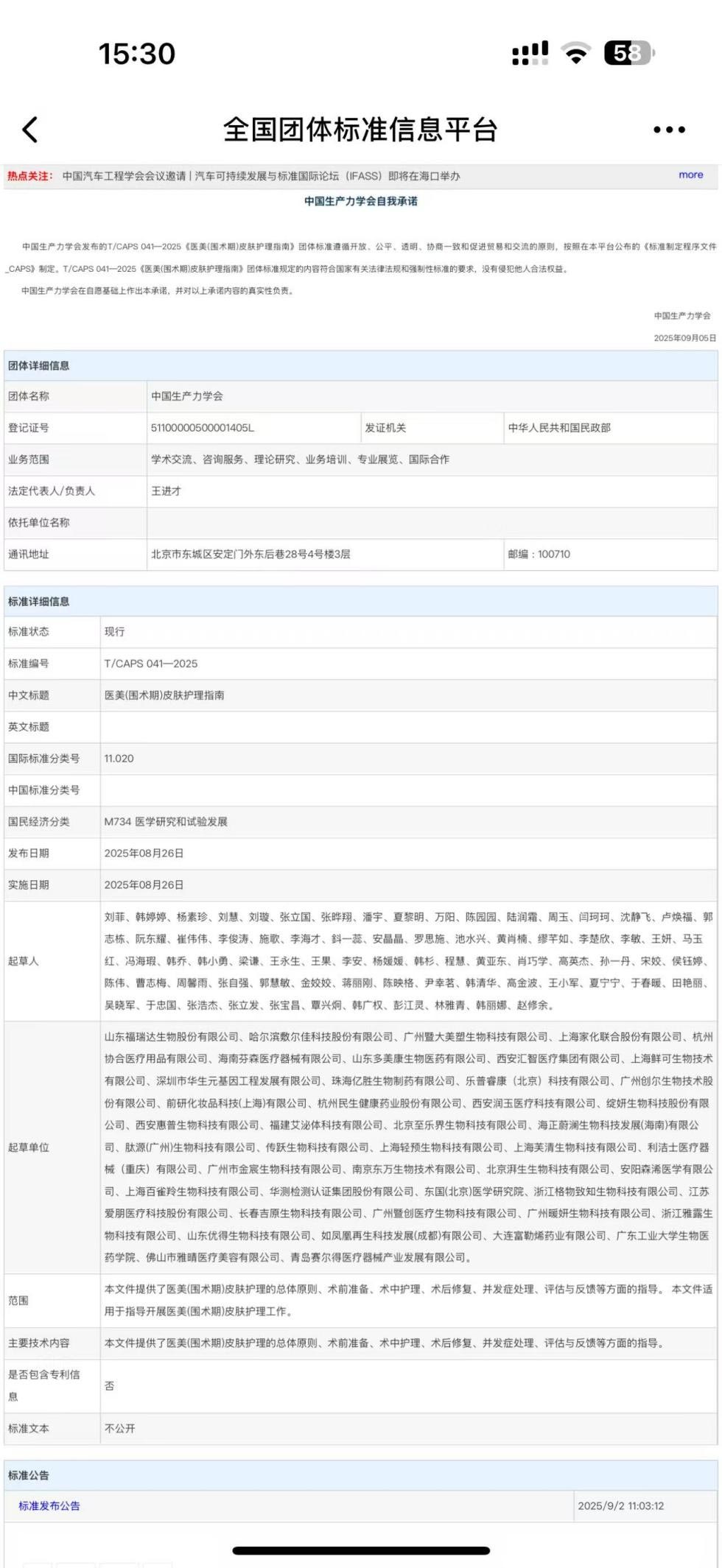
Saildar Medical Participates in the Development of "Medical Beauty (Perioperative) Skin Care Guidelines" Group Standards Officially Released
On August 26, 2025, the group standard "Guidelines for Skin Care in Medical Aesthetics (Perioperative Period)" (standard number: T/CAPS 041 - 2025), jointly drafted by the China Productivity Society and several leading companies in the industry, including Qingdao Saildar Medical Device Industry Development Co., Ltd., was officially released and implemented. This event marks a key step forward in the standardization of my country's medical aesthetics industry and also injects strong momentum into the development of Qingdao Saildar Medical Device Industry Development Co., Ltd.
This standard-setting process brought together the expertise of over 60 organizations nationwide, including medical device companies, biopharmaceutical companies, testing institutions, and research institutions. Saildar Medical, as the only medical device company representative from Qingdao, actively participated in the drafting and content development of the standard, fully demonstrating its professional strength and industry influence in the field of aesthetic medicine care. The standard covers key aspects such as preoperative preparation, intraoperative care, postoperative recovery, complication management, and assessment feedback, providing scientific, standardized, and practical technical guidance for perioperative skin care in aesthetic medicine institutions across the country, filling a standard gap in the field.
Qingdao Saildar Medical Device Industry Development Co., Ltd., though a newcomer to the industry, has demonstrated remarkable development potential and strategic vision. Since its establishment, the company has consistently adhered to the principles of innovation, professionalism, and quality, focusing on the research, development, production, and sales of medical devices, and actively building core competitiveness, especially in the field of aesthetic medical devices.
The newly released group standard is of great significance to Saildar, serving as a bright beacon guiding the company's development. In terms of R&D, Saildar will strictly adhere to the standard's requirements and increase its investment in the research and development of medical devices for perioperative skin care in aesthetic medicine. The company's R&D team will thoroughly analyze the various technical indicators and requirements in the standard, closely combining market demands and clinical feedback to develop safer, more effective, and compliant products. For example, regarding postoperative recovery, the company is committed to developing medical devices with better repair effects and lower irritation to meet the diverse needs of patients and medical institutions.
In the production process, Saildar will establish a rigorous quality control system based on group standards. From raw material procurement to product manufacturing, inspection, and packaging, the entire process will be strictly implemented according to standards to ensure that every product meets high-quality standards. This will not only help enhance the company's product market competitiveness but also provide consumers with reliable medical protection.
The group standard also provides strong support for Saildar's market expansion. As the medical aesthetics market continues to expand, consumers are paying increasing attention to the safety and standardization of medical aesthetic services. With its standard-compliant products, Saildar can better meet market demands and establish a positive brand image. In collaborations with medical institutions, the standardization and professionalism of the company's products will become a key competitive advantage, helping to expand sales channels and increase market share.
Saildar understands the importance of industry exchange and cooperation and has always actively participated in industry activities, sharing experiences and exchanging technologies with peers. Taking advantage of the release of this group standard, the company will further strengthen cooperation with upstream and downstream enterprises. This includes collaborating with raw material suppliers to ensure the quality and stable supply of raw materials; and partnering with medical institutions to conduct clinical research and application promotion, continuously optimizing product performance.
Looking to the future, Qingdao Saildar Medical Device Industry Development Co., Ltd. will be guided by group standards and continue to innovate and develop. The company plans to launch more new products that meet market demands and industry standards in the coming years, and actively expand into domestic and international markets. At the same time, it will strengthen talent cultivation and team building to enhance the company's overall strength and innovation capabilities.
Amidst the rapid development of the medical device industry, Qingdao Saildar Medical Device Industry Development Co., Ltd. is forging ahead with firm steps, leveraging group standards to strive towards becoming an industry leader and contributing to the standardization and professionalization of the medical aesthetics and medical device industry. It is believed that in the near future, Saildar will undoubtedly shine even brighter in the medical device field, becoming a mainstay of industry development.